Conduction band and valence bands overlap in
Right Answer is:
Conductor
SOLUTION
An energy band is a large group of orbitals whose energies are closely spaced and whose average energy is the same as the energy of the corresponding orbital in an individual atom.
An energy band containing valence electrons is called the valence band. If the valence band is partially filled it requires very little added energy to excite a valence electron to a slightly higher energy orbital. Such a small increment of energy can be provided by applying an electric field, for example.
The band with the next higher average energy above the valence band is called the conduction band. In a metal, the valence band and the conduction band overlap, so electrons can move freely from the valence band to the conduction band this explains the high electrical conductivity of metals.
A substance with overlapping valence and conduction bands is an electrical conductor (or just conductor).
When the conduction band is close in energy to the valence band, the electrons can absorb a wide range of wavelengths in the visible region of the spectrum. As the excited electrons fall back to their lower energy states, they emit their extra energy as visit light, producing the luster characteristic of metals.
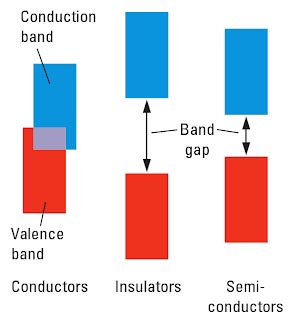
In a semiconductor, a narrow energy gap separates the valence band and the conduction band. At very low temperatures, electrons remain in the filled low energy valence band, and semiconductors are not good conductors. As temperature rise more electrons have enough energy to jump across the band gap into the conduction band, so conduction increases. A sufficiently large electric field also can provide the energy needed for electrons to jump the band gap. This property of semiconductors— switch from insulator to conductor with the application of an external electric field— the basis for the operation of transistors, the cornerstone of modern electronics.
The energy-band theory also explains why some solids are electrical insulators (or just insulators), that is, substances that do not conduct electricity. In an insulator, there is a large band gap between the valence and conduction bands. Very few electrons have enough energy to move across the large gap from a filled lower energy band to an empty higher energy band, so no current flows through an insulator when an external electric field is applied.
