The number of atoms per unit cell in the F.C.C. structure is
The number of atoms per unit cell in the F.C.C. structure is
Right Answer is:
4
SOLUTION
One of the close-packed structures is the face-centered cubic structure. The face-centered cubic structure has atoms located at the eight corners and the centers of all the cubic faces as shown in Fig.
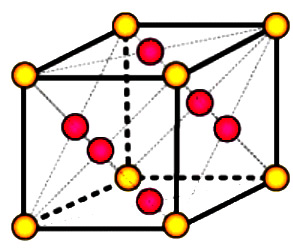
- Each of the corner atoms is the corner of eight cubes so the corner atoms are shared among eight unit cells. Additionally, each of its six face-centered atoms is shared with an adjacent unit cell as shown in Fig.
- It has a coordination number of 12.
- The FCC unit cell consists of a net total of four atoms; one eight of eight corner atoms and six halves of the face-centered atoms.
Number of atoms per unit cell
8 corners × 18 per corner atom = 8 × 18 = 1 atom
6 face-centered atoms × 12 atoms per unit cell = 3 atoms
Hence, the total number of atoms in a unit cell = 3+ 1 = 4 atoms
